Solar Cell
A solar cell, also known as a photovoltaic cell, is an electronic device that converts light energy directly into electrical energy through the photovoltaic effect.
All solar cell materials used till date are semiconductors in crystalline or amorphous forms. A common characteristic of these materials is that they possess a band gap i.e. a discontinuity or rather a range of forbidden values in the energy spectrum. Mostly, solar cells are fabricated from silicon single crystals; Silicon is not transparent for visible light. Therefore, the surface layer of the cell, which is of p type, is made extremely thin to enable maximum light to penetrate the junction. It is desired the absorption of light takes place at the junction region such that the generated electron holes pairs can be separated by the junction fields before they are lost by recombination. To enhance the transmission of the light into the material an anti-reflection coating is given over p type layer. Thin metallic films vacuum deposited suitably on both the sides of the cell act as electrodes. An open circuit voltage of peak value of 0.6 V is generated by a solar cell. Silicon wafer of 1”dia to 4”dia are used to fabricate solar cells. In order to enhance the total voltage and current output, a number of P-n junction are formed on a wafer, using a mesh type or finger like electrode structure. To increase power output, solar cells are arrayed into a series chain or parallel chain and are interconnected. Such an arrangement is called a solar panel. In normal use single solar cell is rarely used, as its output is very low.
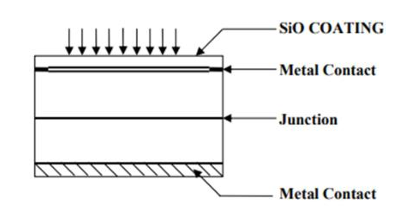
Solar cells are basically solid-state devices. It is basically a p-n junction, which converts sunlight (solar energy) into electrical energy through a three-step process:
1. Generation of carrier pairs (electron hole pairs).
2. Separation of electrons and holes.
3. Collection of separated carriers.


